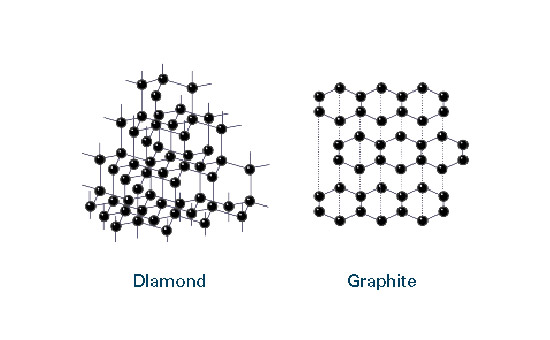
What are diamond and graphite in relation to carbon?
By A Mystery Man Writer
Description
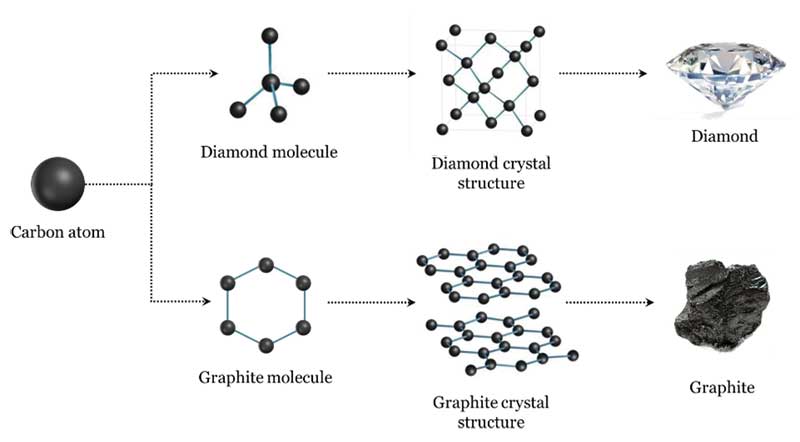
They're both carbon allotropes, however they are arranged differently. Diamond and graphite are both allotropes of carbon. Allotropes are basically different forms of the same element. The only difference is the structure and arrangement of how the carbon atoms are oriented. As you can see, graphite is arranged in a sheet-like arrangement and when used in pencils, sheets of graphite are removed when writing. As for diamonds, they are arranged in a geometric, 3D shape. This is the reason why they are considered the hardest, natural compound. Hope this helps :)

Siyuan Yue - Difference of Graphite and Diamond
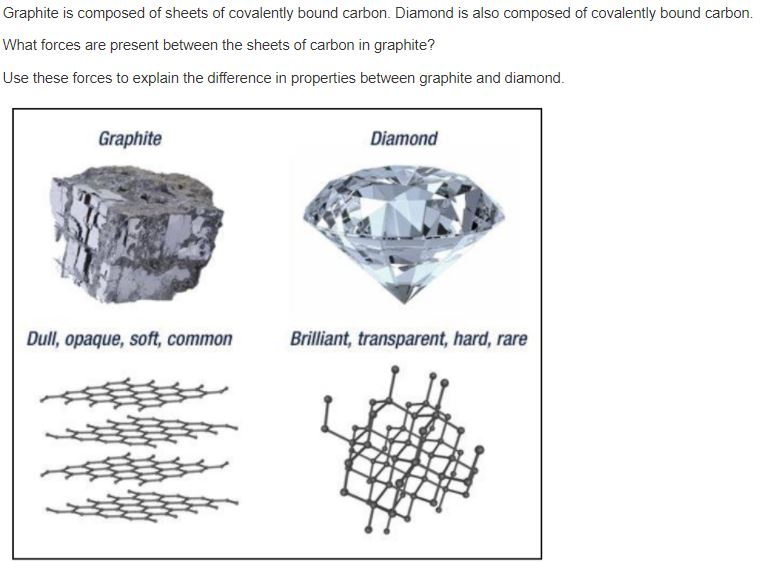
Solved Graphite is composed of sheets of covalently bound
The history of laboratory-grown diamonds - Jewellery Business
What Is Carbon Graphite?

Let's learn about diamond

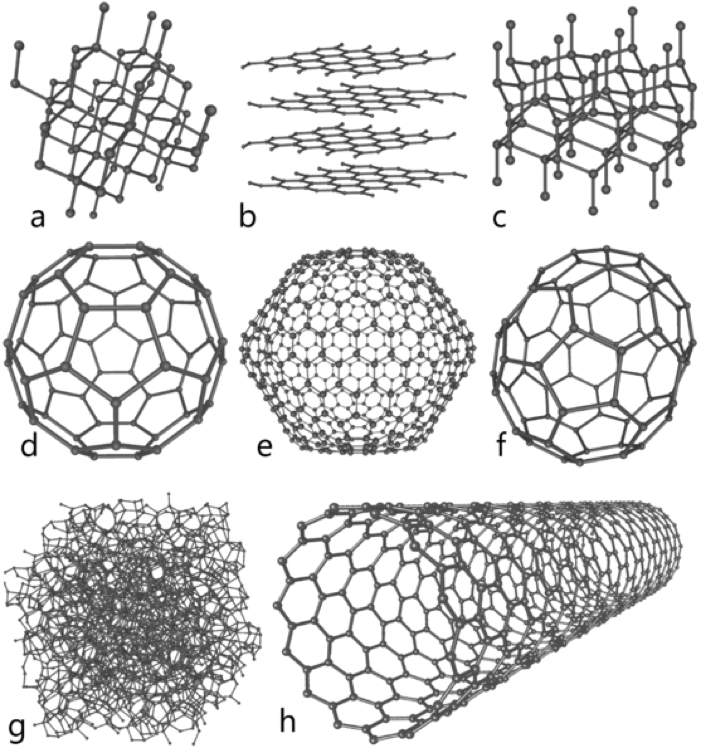
A concise review of the Raman spectra of carbon allotropes

Graphite Graphite is made up of carbon atoms. Chemical formula: C

Distinctive Properties Of Graphite Mining Companies
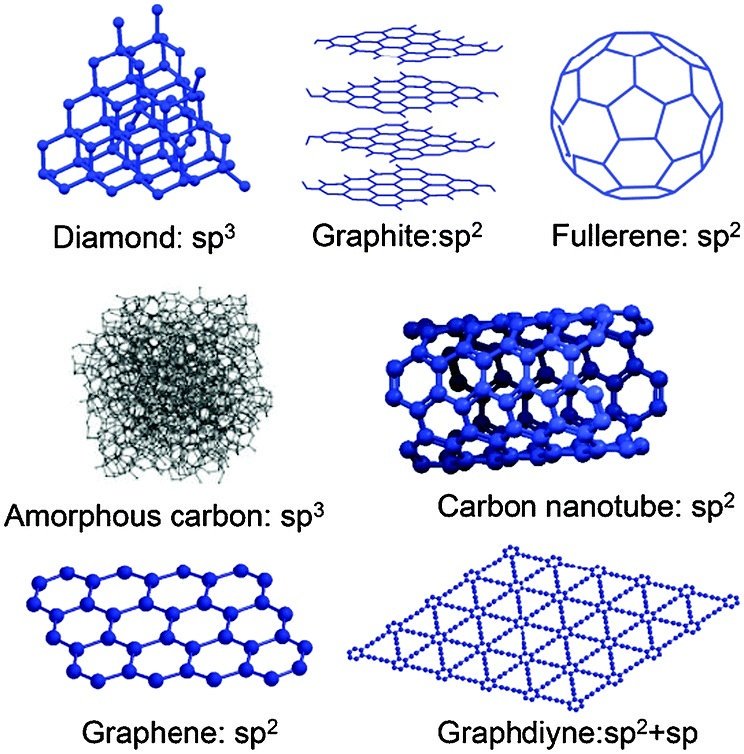
EngineeringSolutions on X: #carbon #allotropes #diamond #graphite

Structure Of Diamond And Graphite
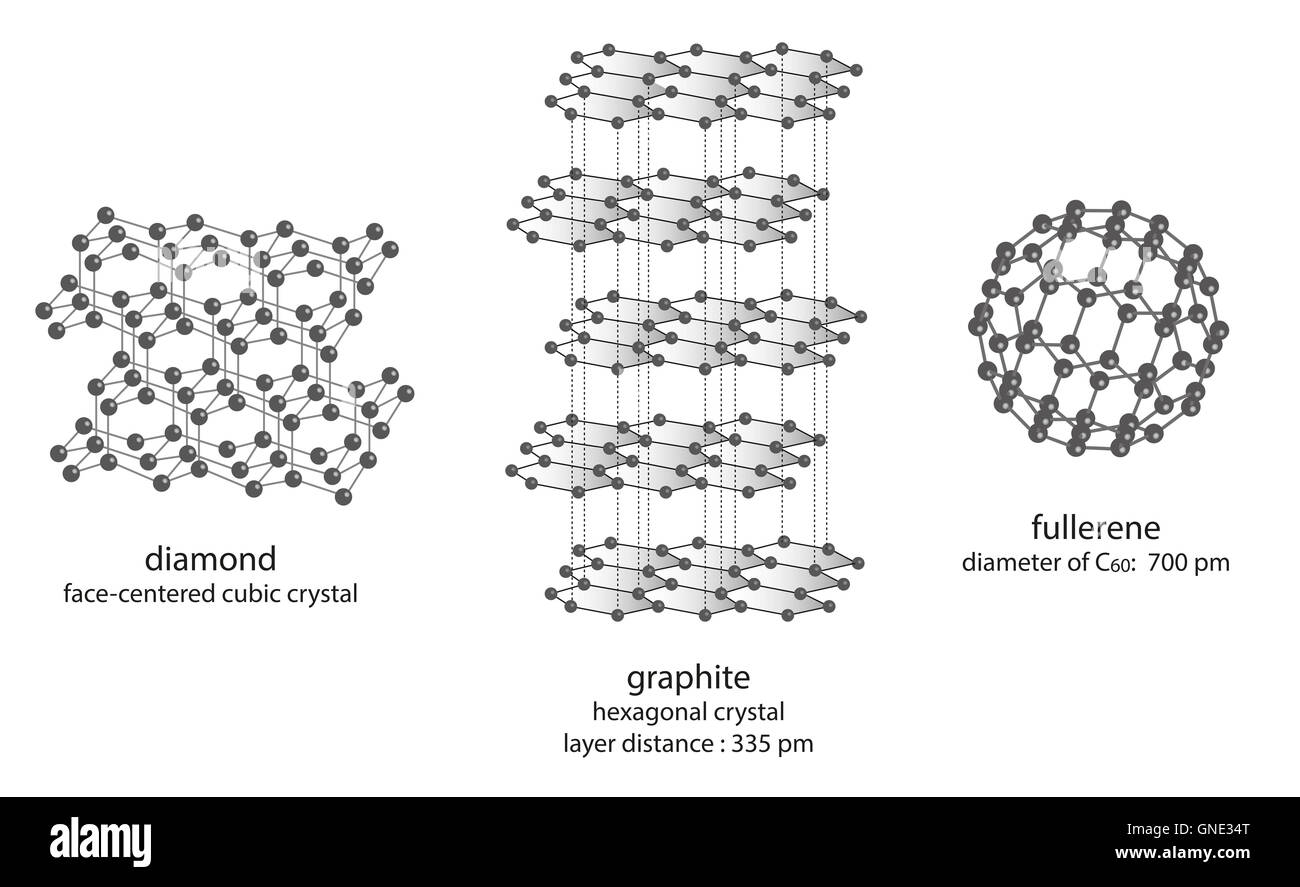
modification of carbon - molecule structure of diamond, graphite
from
per adult (price varies by group size)